Archive: 2026/01 - Page 2
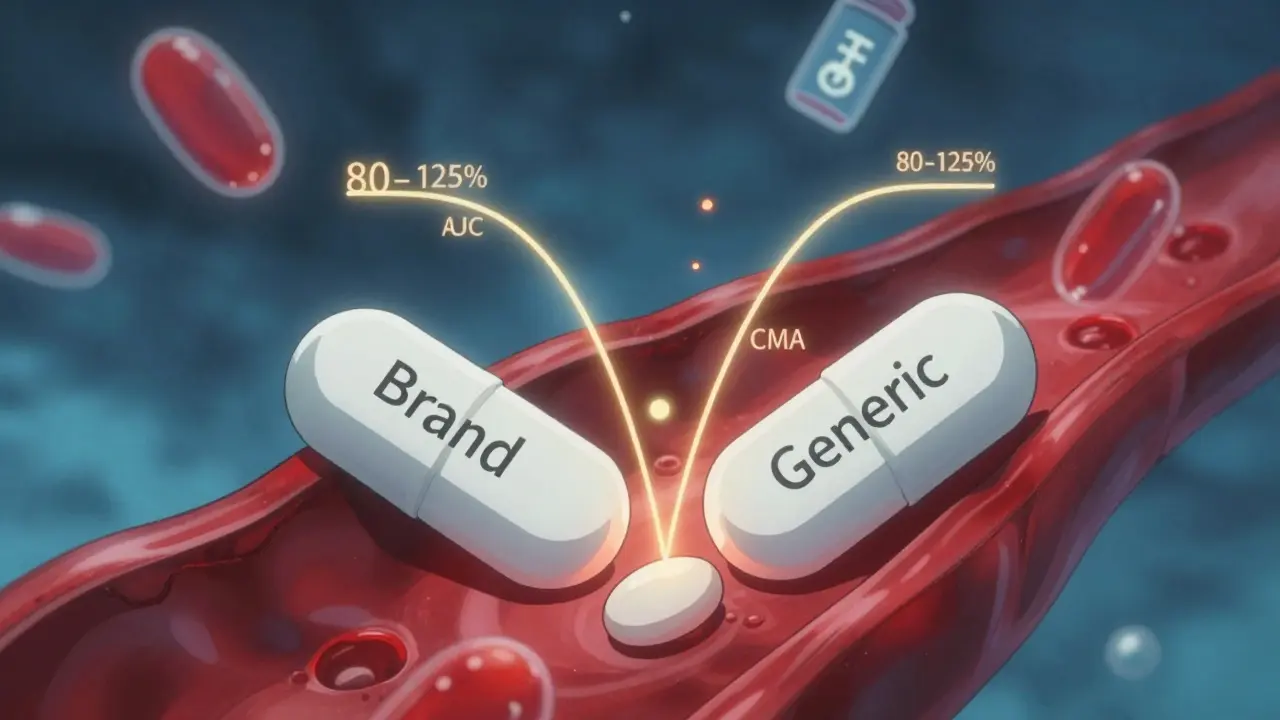
Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
The FDA requires generic drug manufacturers to prove bioequivalence through strict pharmacokinetic studies, ensuring their products match the brand-name drug in absorption and effectiveness. Learn how the 80-125% rule, biowaivers, and product-specific guidelines ensure safety.
Read More
How to Discuss Expired Medication Use during Disasters or Shortages
Learn when and how to safely use expired medications during disasters or drug shortages, based on FDA guidelines, real-world data, and expert recommendations. Know which drugs are safe and which are dangerous.
Read More
Childhood Obesity Prevention and Family-Based Treatment: What Works and Why
Family-based treatment is the most effective way to prevent and treat childhood obesity. Learn how the Stoplight Diet, daily activity, and parent-led behavior changes help kids lose weight-and keep it off-for good.
Read More
Common OTC Medications: Uses, Side Effects, and Safety Information
Learn how to safely use common OTC medications like acetaminophen, ibuprofen, and antihistamines. Understand their uses, side effects, and when to avoid them for better health outcomes.
Read More
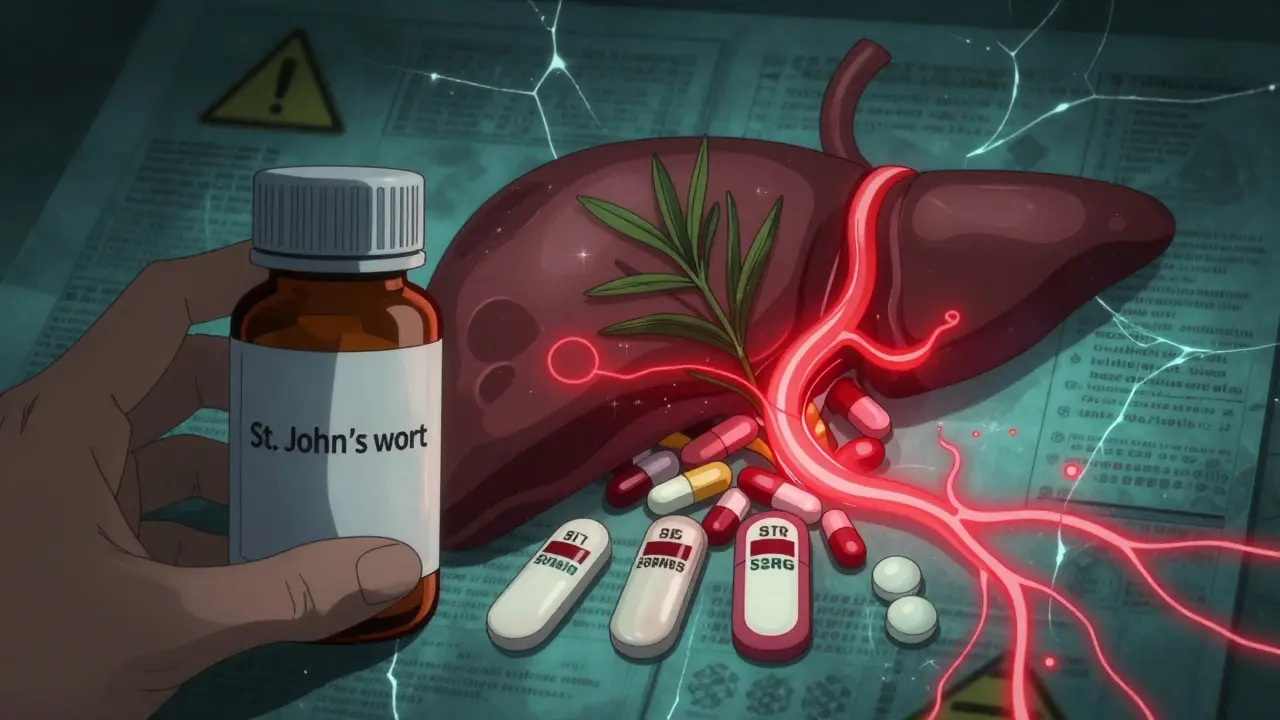
St. John’s Wort and Prescription Drug Interactions: What You Need to Know
St. John’s Wort may help mild depression, but it can dangerously reduce the effectiveness of warfarin, birth control, HIV meds, and more. Learn which drugs it interacts with and what to do if you’re taking it.
Read More
Pneumonia Types: Bacterial, Viral, and Fungal Lung Infections Explained
Learn the key differences between bacterial, viral, and fungal pneumonia-how they start, how they’re diagnosed, and how they’re treated. Knowing the type saves lives and stops unnecessary antibiotic use.
Read More
Generic Drug Patents: How Exclusivity Periods Vary Across Countries
Generic drug exclusivity periods vary widely across countries, affecting how quickly affordable versions reach patients. The U.S. uses complex patent stacking and 180-day incentives, while the EU follows a clearer 8+2+1 model. These rules shape drug prices, innovation, and global access.
Read More
How to Document Safety Alerts on Your Medication List for Better Patient Safety
Document safety alerts on your medication list to prevent life-threatening errors. Learn how to identify high-risk drugs, write exact warnings, and update your list monthly for maximum safety.
Read More
Allergic Reactions to Generics: When to Seek Medical Care
Generic medications can trigger allergic reactions due to differences in inactive ingredients like dyes, lactose, or gluten - even if the brand-name version was safe. Learn the warning signs and when to seek emergency care.
Read More
Feverfew and Anticoagulants: What You Need to Know About Bleeding Risk
Feverfew may increase bleeding risk when taken with anticoagulants like warfarin or apixaban. Learn the signs, the science, and what to do if you're using both.
Read More