When a brand-name drug hits the market, it doesn’t stay alone for long. Somewhere down the line, a cheaper generic version will show up-unless the company behind it has figured out how to delay that day. The rules governing when generics can enter the market aren’t the same everywhere. In the U.S., it’s a legal maze. In the EU, it’s more like a timed track. And in some countries, the wait can stretch far longer than you’d expect.
How Long Do Drug Patents Last?
At first glance, the answer seems simple: 20 years. That’s the standard patent term under the TRIPS Agreement, signed by nearly every country in the world. But here’s the catch-patents are usually filed years before a drug even gets approved. By the time the FDA or EMA gives the green light, 8 to 12 years may already have passed. That leaves innovators with only 6 to 10 years of real market control before generics can legally copy the drug.
That’s why countries built extra layers on top of patents. These aren’t patents-they’re called exclusivity periods. They don’t extend the patent itself. Instead, they block generic companies from using the original drug’s clinical data to get their own approval. Think of it as a legal wall around the drug’s proof of safety and effectiveness.
The U.S. System: A Complex Web of Protections
The U.S. uses the Hatch-Waxman Act of 1984 as its backbone. It’s designed to balance innovation and access, but in practice, it’s become a battleground. The system gives innovators multiple types of exclusivity, often stacking them on top of each other.
- New Chemical Entity (NCE) exclusivity: 5 years. No generic can even apply during this time.
- Orphan Drug Exclusivity: 7 years for drugs treating rare diseases.
- 3-year exclusivity: For new clinical studies on existing drugs-not new ingredients, but new uses or formulations.
- Patent Term Extension (PTE): Up to 5 extra years, but the total protected time after approval can’t go beyond 14 years.
- 180-day exclusivity: The first generic company to challenge a patent gets a head start-no other generics can enter for six months.
Here’s where it gets messy. Brand companies often file dozens, sometimes over a hundred, patents on a single drug. These cover everything from the pill’s coating to the way it’s taken. The FDA lists them in the Orange Book. Generic makers must challenge each one in court. The first to win gets the 180-day window. But some brand companies pay generics to delay entry-called “pay-for-delay.” Courts have cracked down, but it still happens.
One drug, Keytruda, had its effective market protection stretched from 8.2 years to 12.7 years through patent stacking and exclusivity tricks. That’s not rare. Harvard’s Dr. Aaron Kesselheim found that, on average, originator companies file 38 extra patents per drug just to keep generics out longer.
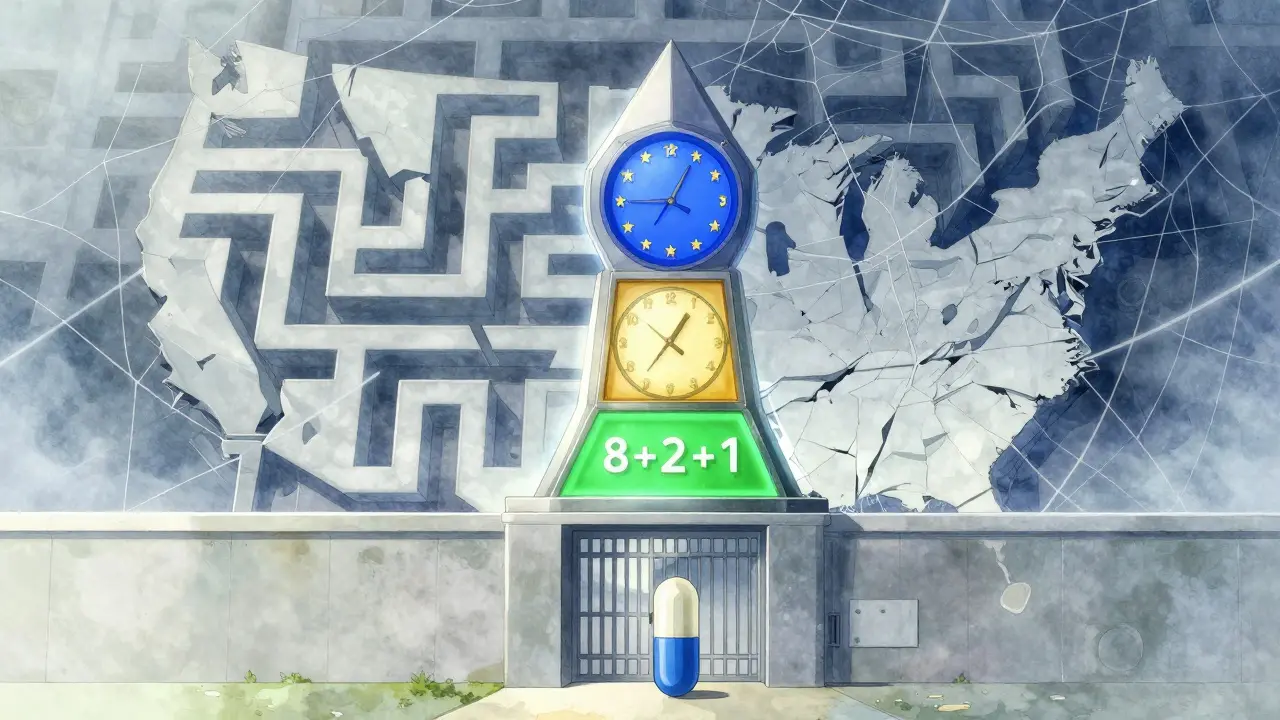
The EU System: The 8+2+1 Rule
The European Union takes a different approach. Instead of multiple overlapping protections, it uses a cleaner, more predictable system: 8+2+1.
- 8 years of data exclusivity: Generic companies can’t use the brand’s clinical trial data to apply for approval.
- 2 years of market exclusivity: Even if a generic gets approved, it can’t be sold during this time.
- 1-year extension: If the brand company adds a new, significant medical benefit during the first 8 years, they get an extra year.
On top of that, the EU offers Supplementary Protection Certificates (SPCs), which can add up to 5 more years of protection-but the total time from drug approval can’t exceed 15 years. That’s less than what’s possible in the U.S., but it’s more transparent.
There’s no 180-day exclusivity for first challengers in the EU. No patent linkage system either. Generic companies don’t have to fight patents before entering the market. That makes entry faster-but also less financially rewarding for the first mover. As a result, fewer generic companies take the risk.
Other Countries: Who’s Following Whom?
Canada mirrors the EU with 8 years of data protection and 2 years of market exclusivity. Japan is stricter: 8 years of data exclusivity, but 4 years of market exclusivity. That’s longer than the EU’s 2-year wait.
China changed the game in 2020, extending data exclusivity from 6 to 12 years. Brazil followed in 2021 with 10 years. These moves were meant to attract big pharma investment, but they’ve also delayed access to affordable drugs in middle-income countries.
In low-income nations, the wait is even longer. The WHO found that essential medicines take an average of 19.3 years to go generic in these countries-over 6 years longer than in high-income ones. Why? Because trade deals often force them to adopt U.S.- or EU-style exclusivity rules, even when they can’t afford the high prices.
Why Does This Matter to You?
If you or someone you know takes a chronic medication-say, for diabetes, high blood pressure, or rheumatoid arthritis-you’ve probably seen the price drop when generics arrive. That drop is usually 80% to 90% within a year. That’s billions in savings for patients and insurers.
But if exclusivity is stretched too long, that savings never comes. Take HIV drugs. In South Africa, data exclusivity clauses in EU trade deals delayed generic versions by up to 11 years after patents expired. People died waiting.
On the flip side, without these protections, companies wouldn’t risk spending $2.3 billion on average to develop a single new drug. The failure rate in late-stage trials is 14%. If no one could profit from success, innovation would stall.

What’s Changing?
Pressure is building. In the U.S., the Preserve Access to Affordable Generics and Biosimilars Act is trying to shut down pay-for-delay deals by making them automatically illegal unless proven otherwise. The EU is considering cutting data exclusivity to 5 years for some drugs to speed up generics. Japan is streamlining its patent review process.
But the big players aren’t backing down. Merck, Pfizer, and others argue that without these protections, next-generation drugs won’t get made. The International Federation of Pharmaceutical Manufacturers & Associations says 97% of its members still see the current system as essential.
Here’s the reality: the system works well for companies that know how to play it. It’s brutal for generic manufacturers trying to cut through legal thickets. And for patients? It’s a gamble. Sometimes, the wait is worth it. Other times, it’s deadly.
What’s Next for Generic Drugs?
Between 2023 and 2028, $356 billion in brand-name drug sales will face patent expiration. That’s a massive opportunity for generics. But the race to get there is harder than ever.
Generic companies now spend $2 to $5 million just to build a legal strategy for one drug. They have to track every patent, every extension, every loophole. One mistake, and they’re stuck for years.
Some are learning to play smarter. Mylan got into the EpiPen market not by copying the original device, but by redesigning it slightly and challenging 6 out of 12 listed patents. That’s how you win now-not just by being first, but by being clever.
Meanwhile, regulators are stuck in the middle. Janet Woodcock, former head of the FDA’s drug center, said it best: ‘We walk a tightrope between innovation and access. Both are critical.’
There’s no perfect system. But as drug prices keep rising and global health gaps widen, the question isn’t whether to change the rules-it’s how fast we can change them before more lives are lost to delays.
How long do generic drugs have to wait before they can enter the market?
It depends on the country and the drug. In the U.S., a new chemical drug gets 5 years of data exclusivity, plus possible patent extensions that can add up to 14 years after approval. In the EU, it’s 8 years of data protection plus 2 years of market exclusivity, with a possible 1-year extension. In Japan, it’s 8 years of data protection and 4 years of market exclusivity. Some drugs get additional protections for rare diseases or pediatric use, which can extend the wait by years.
What is the 180-day exclusivity period in the U.S.?
It’s a reward given to the first generic company that successfully challenges a brand-name drug’s patent under the Hatch-Waxman Act. That company gets a 180-day window where no other generic can enter the market-even if they’ve also won their legal challenge. This incentive was meant to encourage patent challenges, but it’s often exploited through pay-for-delay deals, where brand companies pay generics to delay entry.
Do all countries have the same patent rules for drugs?
No. While the global standard for patent length is 20 years from filing, countries differ in how they handle data exclusivity, market exclusivity, and patent linkage. The U.S. allows stacking of multiple exclusivities and has a complex patent challenge system. The EU uses a fixed 8+2+1 model. Countries like China and Brazil have recently extended exclusivity periods to attract drugmakers, while low-income nations often face longer delays due to trade agreements that force them to adopt stricter rules than they can afford.
What’s the difference between a patent and data exclusivity?
A patent protects the invention-the chemical structure, formulation, or method of use. It’s enforced by courts. Data exclusivity is a regulatory barrier. It stops generic companies from using the brand’s clinical trial data to prove their drug is safe and effective. You don’t need to break a patent to get around data exclusivity-you just can’t use the original data. That’s why data exclusivity often blocks generics even after patents expire.
Why do some generic drugs take longer to appear in low-income countries?
Many low-income countries are pressured by trade deals to adopt U.S. or EU-style data exclusivity rules, even when they can’t afford expensive brand drugs. These rules block generic manufacturers from using clinical data to get approval, even after patents expire. As a result, essential medicines like HIV drugs can be delayed by up to 11 years in places like South Africa. The WHO reports that generic drugs take 19.3 years on average to enter low-income markets-over 6 years longer than in wealthy nations.
Can a drug have both a patent and exclusivity at the same time?
Yes, and that’s common. A drug can have a patent that’s still active while also being protected by data exclusivity. For example, a new drug might have a patent expiring in 2030, but its data exclusivity lasts until 2028. Even if the patent expires in 2030, the generic still can’t enter until 2028 because it can’t use the brand’s clinical data. The exclusivity period often ends first, but the patent can still block generics if it hasn’t expired.

 Epivir (Lamivudine) vs Other HIV Drugs: Detailed Comparison
Epivir (Lamivudine) vs Other HIV Drugs: Detailed Comparison
 Caverta vs Other ED Pills: Detailed Comparison of Sildenafil Alternatives
Caverta vs Other ED Pills: Detailed Comparison of Sildenafil Alternatives
 Wholesale Economics: How Generic Drug Distribution and Pricing Really Work
Wholesale Economics: How Generic Drug Distribution and Pricing Really Work
 Buy Cheap Generic Motrin Online - Safe Tips & Best Deals 2025
Buy Cheap Generic Motrin Online - Safe Tips & Best Deals 2025
Michael Patterson
January 11, 2026 AT 02:54man i swear the us system is just a giant loophole zoo at this point. 5 years here, 7 years there, 180-day monopoly for the first guy who dares to sue, and then the pharma giants just file 40 patents on the color of the pill coating. i read somewhere that one drug had 127 patents listed in the orange book. 127. for one pill. how is this even legal? we’re not protecting innovation, we’re just buying time until the next billionaire’s yacht is paid off. and dont even get me started on pay-for-delay. its basically bribery with a law degree.
Priscilla Kraft
January 12, 2026 AT 07:13ugh i feel you 😩 but honestly, i think we need both sides. yes, the system is broken, but without those protections, companies would never spend $2.3B on a drug that 90% of the time fails in trials. maybe the fix isn’t to remove exclusivity, but to cap how many patents you can stack? like, max 5 per drug? and ban pay-for-delay outright? 🤔
Christian Basel
January 13, 2026 AT 12:29the regulatory arbitrage is staggering. us employs a patent-thicketing strategy with non-transparent exclusivity stacking, whereas eu adopts a time-bound, segmented data + market exclusivity regime. the result? us has longer de facto monopolies but higher litigation costs; eu has lower entry velocity but greater predictability. it’s a classic trade-off between innovation incentive and market efficiency.
Adewumi Gbotemi
January 13, 2026 AT 22:26in nigeria, we wait 10+ years for cheap meds even after patents expire. why? because our government signed deals to copy usa rules. people die waiting. this isn’t about innovation-it’s about who gets to live.
Jennifer Littler
January 14, 2026 AT 13:15the 8+2+1 eu model is actually genius. simple, transparent, no legal minefield. why can’t the us just adopt that? oh right-lobbying. $3.4 billion spent by pharma on lobbying in 2023 alone. the system isn’t broken because it’s flawed-it’s broken because it’s working exactly as intended for the 1%.
Vincent Clarizio
January 15, 2026 AT 16:21let’s be real. the entire pharmaceutical industry is a cult of intellectual property worship. they don’t cure diseases-they monetize suffering. you think they care if a child in india dies waiting for a $1000 HIV drug? no. they care if their quarterly earnings dip. and we let them. we let them patent the damn air if they can. this isn’t capitalism-it’s medical feudalism. the only thing more tragic than the delay is how calmly we accept it.
Sam Davies
January 16, 2026 AT 05:58so the us gives you 14 years of market control after approval? wow. that’s like getting a lifetime supply of free coffee… if you’re the only one allowed to brew it. meanwhile, the eu says ‘8 years data, 2 years market, done’. sounds fair. guess who’s got the better deal? the guy with the money, obviously. the rest of us just get to cheer from the sidelines while our prescriptions cost more than our rent.
Alex Smith
January 16, 2026 AT 19:56fun fact: the first generic to challenge a patent gets 180 days of exclusivity… which sounds like a reward until you realize it’s also a weapon. brand companies pay them to sit on it. so instead of lowering prices, they just… wait. and we pay more. this isn’t competition. it’s collusion with a law degree. also, why is the FDA the referee in a game where one team writes the rules?
Priya Patel
January 18, 2026 AT 05:57my mom takes blood pressure meds and the price dropped from $400 to $12 when generics came in. i cried. but in some countries? that moment never comes. we need global justice, not just local fixes 🌍❤️
Sean Feng
January 19, 2026 AT 20:11Roshan Joy
January 21, 2026 AT 03:52really appreciate this breakdown. one thing missing though-what about biosimilars? they’re not generics, but they’re the next wave. and the rules around them are even messier. in india, we’re starting to make them cheaper, but the us and eu still treat them like new drugs. maybe that’s where real change starts? not with pills, but with biologics?