Archive: 2025/12 - Page 3
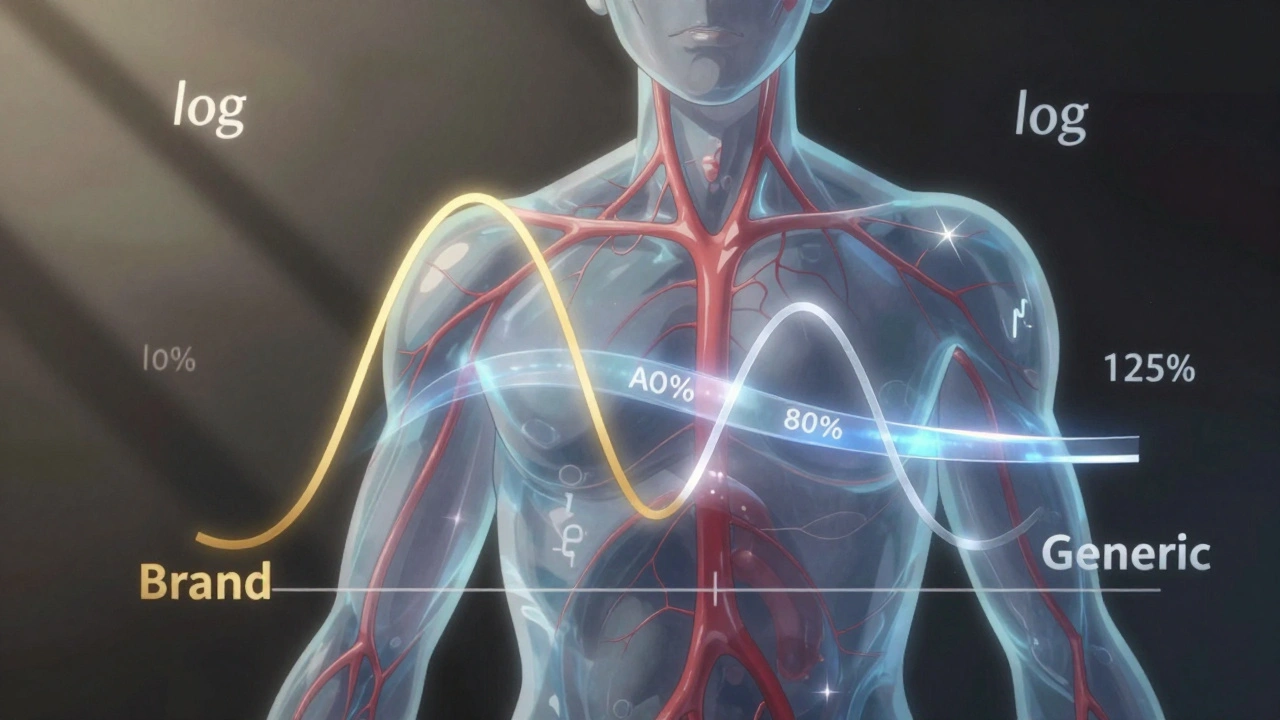
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals for Generic Drugs
The 80-125% rule ensures generic drugs are absorbed the same way as brand-name versions. It's based on pharmacokinetic data and confidence intervals, not active ingredient amounts. Learn how it works and why it matters.
Read More
What Are Biosimilars? A Simple Guide for Patients
Biosimilars are highly similar, FDA-approved versions of complex biologic drugs. They work the same way, have the same safety profile, and cost 15-30% less. Learn how they differ from generics and why they're a safe, affordable option for treating arthritis, cancer, diabetes, and more.
Read More
Symptoms of Taking Counterfeit Meds: What to Watch For
Counterfeit meds can look real but kill you fast. Learn the warning signs-like strange side effects, pills that don’t work, or fentanyl poisoning-and how to protect yourself from fake pills sold online or in pharmacies.
Read More
How to Use Travel Health Clinics for Pre-Trip Medication Planning
Learn how travel health clinics customize vaccines and medications for your trip, from malaria pills to yellow fever shots, and why timing matters more than you think. Get the facts on where to go, what to ask, and how to avoid getting sick abroad.
Read More
Nebulizers vs. Inhalers: Which One Really Works Better for Asthma and COPD?
Nebulizers and inhalers both deliver asthma and COPD medication, but inhalers with spacers are faster, cheaper, and just as effective for most people. Learn who should use what and why.
Read More
Adolescents on ADHD Medications: Growth, Appetite, and Side Effect Monitoring
ADHD medications help teens focus but can suppress appetite and slow growth. Learn how to monitor side effects, when to adjust treatment, and how most teens catch up on growth over time.
Read More
FDA-Approved Medications You Can Flush Down the Toilet (And Which Ones You Shouldn’t)
The FDA allows only a short list of dangerous medications to be flushed down the toilet to prevent accidental overdose. Learn which drugs qualify, when to flush them, and safer alternatives.
Read More
Weekend Weight Gain: How to Stop Calorie Creep and Keep Progress
Weekend weight gain is a common but hidden obstacle in weight management. Learn why calories creep up on weekends, how exercise alone isn't enough, and five simple, proven strategies to stop the cycle without feeling deprived.
Read More
FDA Generic Drug Approval: Step-by-Step Process for ANDA Submission
Learn how the FDA approves generic drugs through the ANDA process - from bioequivalence studies to manufacturing standards and regulatory timelines. Discover what it takes to bring a generic drug to market and why it saves billions annually.
Read More
How Medicines Work and When They’re Safe to Use
Learn how medicines interact with your body at a molecular level and why understanding their mechanism is the key to using them safely. Real examples, practical tips, and science-backed safety rules.
Read More