Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill, you’re counting on one thing: it does the same job as the brand-name version. That guarantee comes down to bioequivalence, the scientific proof that two drugs release the same amount of active ingredient into your bloodstream at the same rate. Also known as therapeutic equivalence, it’s the reason your pharmacist can swap a $200 brand drug for a $5 generic without risking your health. This isn’t guesswork—it’s measured in real people, using blood tests to track how fast and how much of the drug enters your system. If the numbers match within strict FDA limits, the drugs are considered bioequivalent. No magic. No shortcuts. Just hard data.
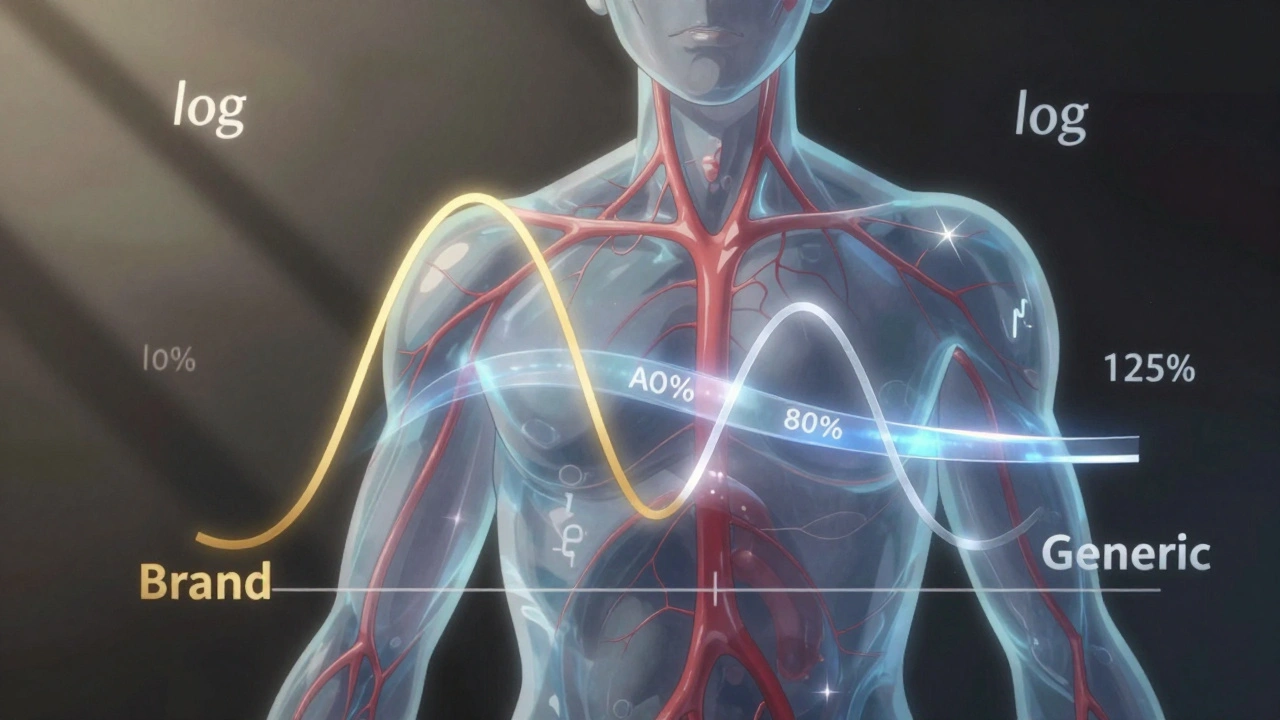
Behind every generic drug approved by the FDA is a bioequivalence study, a controlled trial comparing the generic to the original brand. These studies aren’t done in labs with test tubes—they’re done on healthy volunteers who take both versions, then give blood samples over hours. The results must show the generic delivers 80% to 125% of the brand’s drug levels, and the timing of absorption must be nearly identical. That’s the threshold. Anything outside that range gets rejected. This is why you can trust that a generic metformin for diabetes or a generic lisinopril for high blood pressure works just like the name brand. It’s not cheaper because it’s weaker—it’s cheaper because the manufacturer didn’t spend millions on marketing or patents. And here’s the twist: even if two generics are made by different companies, they both have to prove bioequivalence to the same brand-name drug. That means if Generic A and Generic B both match the brand, they’re also effectively equivalent to each other—even if they look totally different.
But bioequivalence isn’t just about the active ingredient. It’s also about how your body handles the pill. Some people worry that different fillers, dyes, or coatings in generics might cause side effects. That’s where inactive ingredients, the non-medicinal parts of a pill like lactose or titanium dioxide come in. While they don’t affect bioequivalence, they can trigger allergies or interact with other meds. That’s why your pharmacist asks if you’re allergic to dyes or gluten when you pick up a new generic. The drug works the same—but your body might react differently to the extras.
And here’s something most people don’t realize: bioequivalence doesn’t mean identical results for everyone. If you’re on a narrow-therapeutic-index drug—like warfarin, lithium, or thyroid meds—your doctor might stick with the brand because small differences in absorption can matter. But for 90% of medications, including antibiotics, antidepressants, and blood pressure pills, bioequivalence is more than enough. The FDA has approved over 15,000 generic drugs using this standard. Billions of doses are taken every year without issue.
So when you see "generic" on your prescription, know this: it’s not a downgrade. It’s a scientifically verified copy. The same active ingredient. The same effect. The same safety profile. Just a fraction of the cost. And if you’ve ever wondered why your pill changed color or shape after switching pharmacies—that’s trademark law, not quality control. The inside? Still bioequivalent.
Below, you’ll find real stories and facts from patients, pharmacists, and regulators about how bioequivalence plays out in everyday life—from saving money on insulin to avoiding counterfeit pills that pretend to be generics. These aren’t theory pieces. They’re real-world checks on what happens when science meets the medicine cabinet.