When you take a pill once a day instead of three times, it’s not just convenience-it’s science. Modified-release (MR) formulations are designed to control how and when a drug enters your bloodstream. They’re used in everything from blood pressure pills to ADHD medications. But getting these drugs approved as generics isn’t as simple as copying a tablet. There’s a whole layer of testing called bioequivalence that’s far more complex than for regular pills. If you’re wondering why some generic MR drugs work differently than others-or why some get rejected by regulators-this is why.
What Makes Modified-Release Formulations Different?
Immediate-release (IR) drugs dump their entire dose into your system quickly. That’s fine for some medicines, but for others, it causes spikes and crashes in blood levels. Too much at once can cause side effects. Too little later on means the drug stops working. Modified-release formulations solve this by slowly releasing the drug over hours. Think of it like a time-release capsule: one part releases fast, another part drags out over the day.
These aren’t just fancy pills. They’re engineered systems. Some use coatings that dissolve at different pH levels. Others have tiny beads inside a capsule that release at staggered intervals. Some even resist being broken down by alcohol. That last part matters-drinking while on certain extended-release opioids can cause a dangerous surge of drug into the bloodstream. That’s called dose dumping, and it’s led to seven drug withdrawals between 2005 and 2015.
Today, about 35% of all approved generic drugs are modified-release. In the U.S. alone, they make up $65 billion in annual sales. That’s not small change. It’s a huge chunk of the market, and regulators treat them like high-stakes products.
Why Bioequivalence for MR Drugs Isn’t Just About AUC and Cmax
For regular pills, bioequivalence is pretty straightforward: compare the total drug exposure (AUC) and the peak concentration (Cmax) between the brand and generic. If both are within 80-125% of each other, you’re good.
But for MR drugs? That’s not enough.
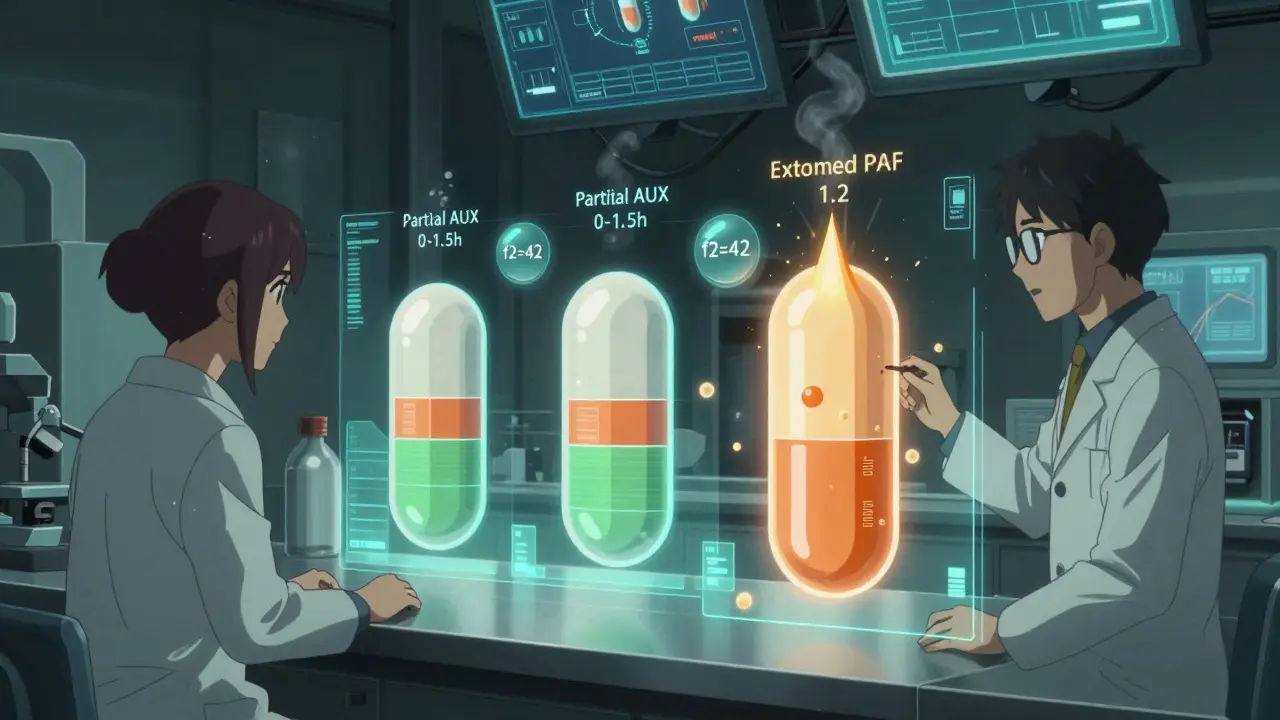
Take a drug like Ambien CR. It has two phases: one that kicks in fast to help you fall asleep, and another that lasts longer to keep you asleep. If the generic only matches the overall exposure but messes up the timing of those two phases, you might wake up in the middle of the night-or feel groggy in the morning. That’s why regulators require partial AUC measurements.
The FDA requires testing two time windows: from zero to 1.5 hours (the fast part) and from 1.5 hours to infinity (the slow part). Both have to be within 80-125%. One failure, and the application gets rejected. In fact, 22% of MR generic applications between 2018 and 2021 were turned down because they didn’t measure the right timepoints.
And it’s not just timing. For drugs with high variability-like warfarin or certain seizure medications-the acceptance range tightens to 90-111%. That’s because even small differences can cause serious harm. A 10% drop in blood levels might mean a seizure. A 10% spike might mean bleeding.
How Regulators Compare MR Products: FDA vs. EMA
The FDA and EMA (Europe’s drug agency) don’t see eye to eye on everything. And that creates real headaches for drugmakers trying to sell globally.
The FDA says: Single-dose studies are better. Why? Because they show how the drug behaves when there’s no buildup. Multiple doses can mask problems with release patterns. Since 2015, 92% of approved extended-release generics used single-dose studies.
The EMA, on the other hand, sometimes still requires steady-state studies-where patients take the drug daily for days or weeks until levels stabilize. They argue this better reflects real-world use, especially for drugs that accumulate.
But here’s the conflict: a 2018 paper by Dr. Lawrence Lesko called the EMA’s steady-state requirement “lacking scientific justification for most products.” Meanwhile, Dr. Donald Mager argues it’s necessary for drugs with high accumulation.
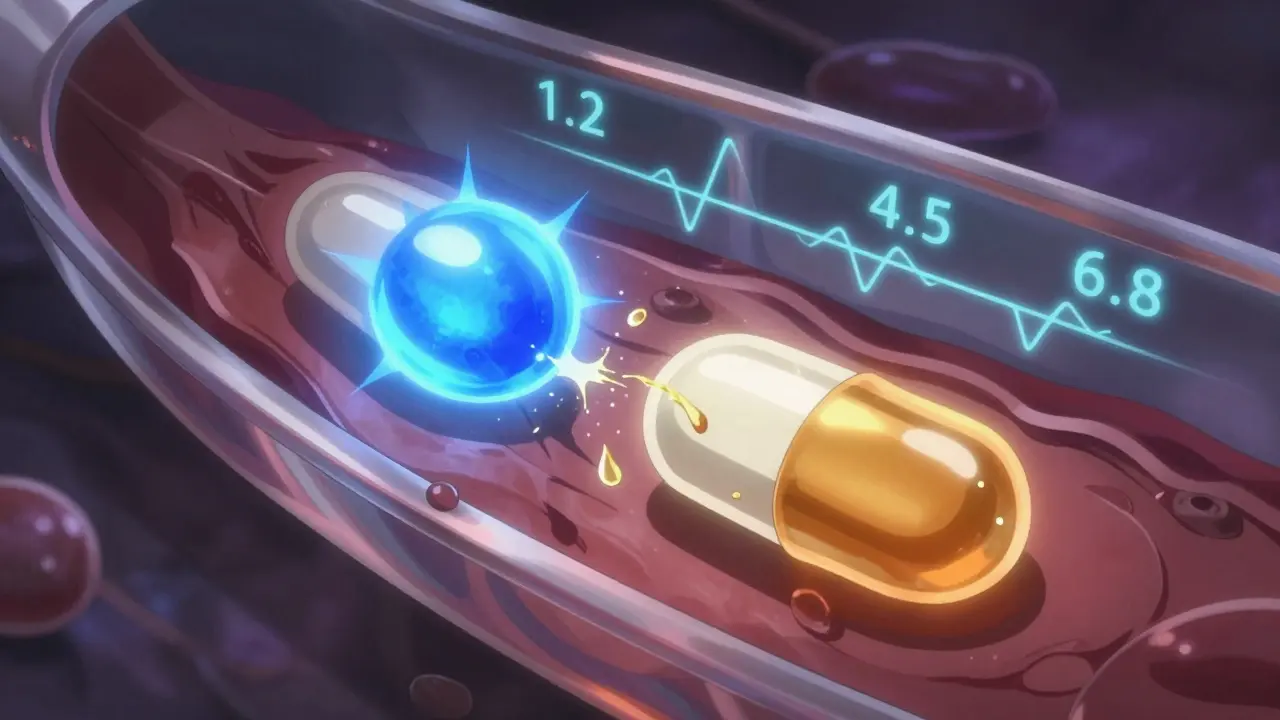
Dissolution testing is another battleground. The FDA demands testing at three pH levels-1.2 (stomach), 4.5 (upper intestine), and 6.8 (lower intestine)-for extended-release tablets. The EMA accepts two. And for beaded capsules? The FDA only requires one condition. That inconsistency means a product that passes in the U.S. might fail in Europe, and vice versa.
The Hidden Costs and Failures Behind Generic MR Drugs
Developing a generic MR drug costs $5-7 million more than a regular one. Why? Because the studies are harder, longer, and more expensive.
A single-dose MR bioequivalence study runs $1.2-1.8 million. Compare that to $0.8-1.2 million for a standard IR study. And it’s not just money. It’s time. For highly variable drugs, using the Reference-Scaled Average Bioequivalence (RSABE) method adds 6-8 months to development. That’s because you need more volunteers, more blood draws, and complex statistical modeling.
And failures are common. One formulation scientist at Teva reported that 35-40% of early-stage oxycodone ER generics failed dissolution testing across the three pH levels. A generic version of Concerta (methylphenidate ER) was rejected in 2012 because it didn’t match the brand’s release profile in the first two hours-critical for ADHD patients who need quick symptom control.
But success stories exist. Sandoz got its extended-release tacrolimus approved using a biowaiver based on dissolution similarity (f2=68 at pH 6.8). That saved $1.5 million and 10 months. How? By showing their tablet released drug at nearly the same rate as the brand under all conditions.
What It Takes to Get It Right
There’s no shortcut. Getting MR bioequivalence right requires:
- Advanced dissolution testing-often using USP Apparatus 3 or 4, not the standard Apparatus 2
- Pharmacokinetic modeling software like Phoenix WinNonlin or NONMEM
- Statistical expertise to handle RSABE and partial AUC calculations
- Understanding of product-specific guidances (PSGs)-the FDA has over 150 of them for MR drugs
And the learning curve? According to a 2022 survey by the International Society for Pharmacokinetics, it takes 12-18 months of training just to become competent in MR BE study design.
Most of this work is done by big pharma and large contract research organizations (CROs). Only 3% of MR BE studies are done by small biotechs. The cost and complexity are just too high.
The Future: IVIVC, PBPK, and What’s Next
The field is moving beyond just testing. The FDA now accepts in vitro-in vivo correlation (IVIVC) models for some MR products. That means if a tablet’s dissolution profile perfectly predicts how it behaves in the body, you might not need a full human study. Twelve products have been approved this way since 2019.
Even more advanced is physiologically based pharmacokinetic (PBPK) modeling. It simulates how a drug moves through the body based on anatomy, pH, blood flow, and enzyme activity. A 2022 DIA survey found 68% of major pharma companies now use PBPK for MR development.
And regulators are catching up. The FDA plans to release a new guidance in 2024 on complex MR systems-like gastroretentive tablets that float in the stomach, or multiparticulate systems with dozens of tiny beads. These are the future of chronic disease management.
By 2028, IQVIA predicts MR formulations will make up 42% of all prescription sales. Aging populations, chronic illnesses, and demand for once-daily dosing are driving this growth. But with growth comes risk. A 2016 study in Neurology found that 18% of generic extended-release antiepileptic drugs had higher seizure rates than the brand-even though they passed standard bioequivalence tests.
That’s the real challenge: meeting the numbers doesn’t always mean matching the experience.
Why can’t we just use the same bioequivalence rules for modified-release drugs as for immediate-release ones?
Because modified-release drugs are designed to release the drug over time, not all at once. If you only compare total exposure (AUC) and peak concentration (Cmax), you might miss critical differences in how fast or when the drug is released. For example, a generic might have the same overall AUC as the brand, but release too much too early or too little too late-leading to side effects or loss of effectiveness. That’s why regulators require partial AUC measurements and dissolution profile matching.
What is dose dumping, and why is it a concern with extended-release drugs?
Dose dumping happens when alcohol or other substances cause an extended-release drug to release its entire dose at once instead of gradually. This can lead to dangerous spikes in blood levels. For example, drinking while taking certain opioid painkillers can cause overdose. The FDA requires alcohol interaction testing for all ER products containing 250 mg or more of active ingredient. Between 2005 and 2015, seven such products were pulled from the market because of this risk.
Why do some generic MR drugs work differently than the brand even if they’re bioequivalent?
Bioequivalence standards focus on average blood levels, not individual patient response. Some patients are more sensitive to timing or small variations in release. A 2016 study found that 18% of generic extended-release antiepileptic drugs had higher seizure rates than the brand, even though they passed standard bioequivalence tests. This suggests that current methods may not capture all clinical differences, especially for drugs with narrow therapeutic windows.
How do FDA and EMA guidelines differ on bioequivalence testing for MR drugs?
The FDA primarily uses single-dose fasting studies and requires partial AUC measurements for multiphasic drugs. It also demands dissolution testing at three pH levels (1.2, 4.5, 6.8) for tablets. The EMA sometimes requires steady-state studies and focuses more on metrics like half-value duration and midpoint duration time. It also accepts a lower f2 similarity factor for biowaivers and doesn’t always require three pH tests. These differences mean a product approved in the U.S. might not be approved in Europe, and vice versa.
What is RSABE, and why is it used for some MR drugs?
RSABE stands for Reference-Scaled Average Bioequivalence. It’s used for highly variable drugs-where the brand’s own blood levels vary a lot between patients (within-subject CV >30%). Standard 80-125% limits are too strict here, so RSABE widens the range based on the brand’s variability, up to a cap of 57.38%. This prevents rejecting good generics just because the drug is naturally inconsistent. It’s required for drugs like warfarin, clopidogrel, and some antiepileptics.
Can a generic MR drug get approved without doing a human bioequivalence study?
Yes, under certain conditions. If the drug’s dissolution profile matches the brand across all required pH levels and conditions, and if there’s a validated in vitro-in vivo correlation (IVIVC), regulators may grant a biowaiver. This is rare and only applies to a few products, like some extended-release tacrolimus and paliperidone formulations. It saves time and money, but requires extensive lab data and modeling.
What’s the biggest challenge in developing a generic modified-release drug?
The biggest challenge is replicating the release profile exactly. Even small changes in coating thickness, bead size, or excipient composition can alter how the drug releases. Many developers fail because their dissolution profile doesn’t match the brand across all three pH levels. It’s not just about the active ingredient-it’s about the entire delivery system. That’s why 35-40% of early-stage oxycodone ER generics fail initial testing.
 How and Where to Buy Keppra Online: Safe Ordering & Tips
How and Where to Buy Keppra Online: Safe Ordering & Tips
 Can Tazarotene Reduce Wrinkles? Anti‑Aging Benefits Explained
Can Tazarotene Reduce Wrinkles? Anti‑Aging Benefits Explained
 How to Report Suspected Counterfeit Drugs to Authorities
How to Report Suspected Counterfeit Drugs to Authorities
 Grifulvin V: Uses, Side Effects, and Essential Tips for Antifungal Treatment
Grifulvin V: Uses, Side Effects, and Essential Tips for Antifungal Treatment
 Modified-Release Formulations: What You Need to Know About Bioequivalence Standards
Modified-Release Formulations: What You Need to Know About Bioequivalence Standards