For years, hepatitis C was a slow-burning crisis-silent, stubborn, and hard to beat. But today, thanks to drugs like velpatasvir, curing hepatitis C isn’t just possible-it’s routine. In 2025, over 95% of people treated with velpatasvir-based regimens clear the virus in just 8 to 12 weeks. No more interferon shots. No more months of nausea and fatigue. Just a daily pill, a short course, and a clean bill of health.
What Is Velpatasvir?
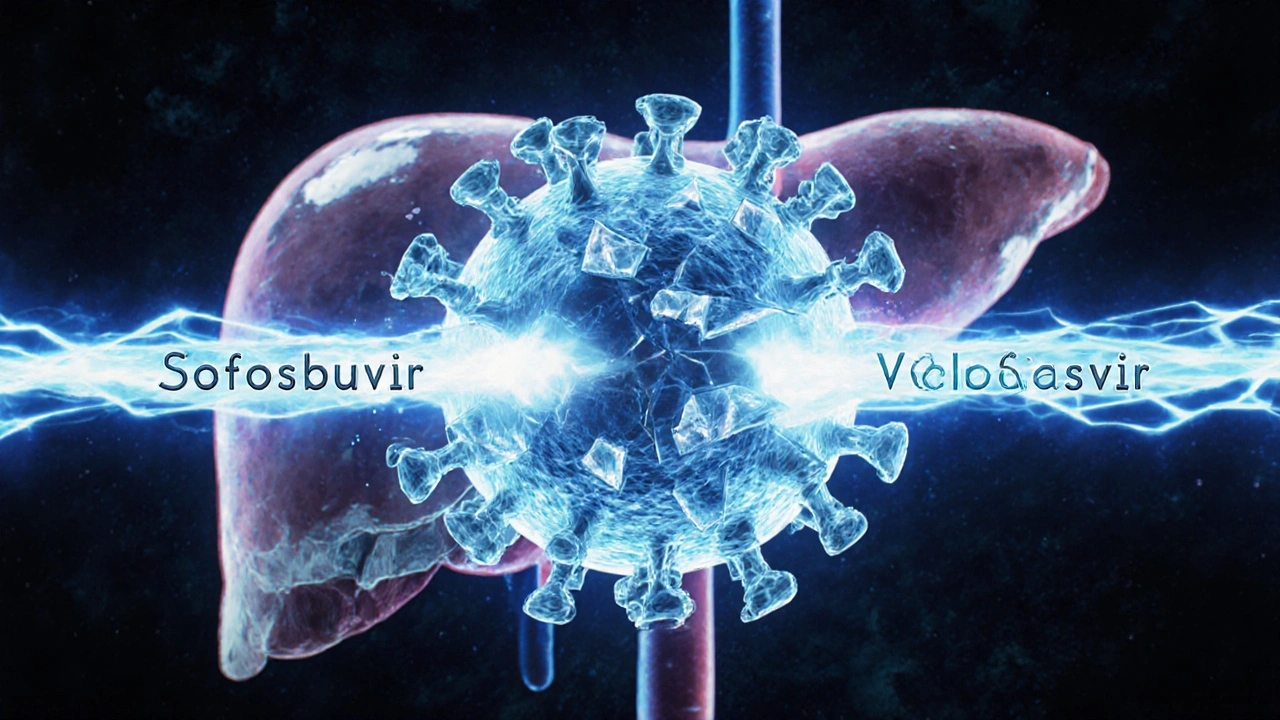
Velpatasvir is a direct-acting antiviral (DAA) that blocks the NS5A protein, a key piece of the hepatitis C virus’s machinery. Without NS5A, the virus can’t copy itself or spread to new liver cells. It doesn’t work alone-it’s always paired with another drug, sofosbuvir. Together, they form the combination pill known as Epclusa.
Before velpatasvir, treatments varied by hepatitis C genotype. There were six major strains, and each needed a different combo of drugs. Some regimens lasted 24 weeks. Others had harsh side effects. Velpatasvir changed that. It’s the first single-pill treatment that works against all six genotypes. That means a patient in rural Ohio or a refugee camp in Kenya can get the same effective treatment without needing expensive lab tests to identify their exact strain.
How Velpatasvir Changed the Game
In 2016, when Epclusa (sofosbuvir/velpatasvir) was first approved by the FDA, it was hailed as a breakthrough. But the real impact came later-when health systems started using it at scale. A 2023 study from the CDC tracked over 12,000 patients treated with Epclusa across U.S. safety-net clinics. The cure rate? 97%. Even in people with cirrhosis, HIV co-infection, or prior treatment failure, the numbers stayed above 90%.
What made this possible? Velpatasvir’s high barrier to resistance. Unlike older DAAs, the virus rarely mutates to escape it. That’s why doctors now consider Epclusa the first-line treatment for nearly every hepatitis C patient, regardless of liver damage or medical history.
Real-World Impact: From Diagnosis to Cure
Before velpatasvir, many people with hepatitis C never got treated. The process was too complicated. You needed multiple blood tests, specialist referrals, and sometimes years on a waiting list. Now, primary care doctors in Massachusetts, Texas, and Alaska can prescribe Epclusa after a simple blood test. No hepatologist needed.
In Boston, the Massachusetts General Hospital launched a community-based screening program in 2022. They offered free hepatitis C tests at pharmacies, homeless shelters, and needle exchange programs. Of the 1,800 people tested, 212 were positive. All 212 were started on Epclusa. By the end of 2024, 207 had been cured. That’s a 98% success rate in a population often ignored by traditional healthcare.
What’s New in 2025?
The biggest update isn’t a new drug-it’s a new approach. In early 2025, the WHO updated its global guidelines to recommend a single 8-week course of sofosbuvir/velpatasvir for most adults, even those with advanced liver disease. Previously, 12 weeks was standard. Now, shorter treatment means lower cost, better adherence, and faster results.
Researchers are also testing velpatasvir in new combinations. One Phase 3 trial in 2024 paired velpatasvir with a new drug called voxilaprevir in patients who failed prior DAA therapy. The cure rate hit 96% after just 12 weeks. That’s huge for the 5-10% of patients who don’t respond to first-line treatment.
Another breakthrough: generic versions. In 2023, India and Egypt began producing low-cost generics of Epclusa. By 2025, the price per treatment course dropped from $75,000 in the U.S. to under $50 in low-income countries. Global health groups are now shipping these generics to sub-Saharan Africa and Southeast Asia. The goal? Eliminate hepatitis C as a public health threat by 2030.
Who Should Take Velpatasvir?
Velpatasvir (in Epclusa) is approved for adults and children over 6 years old with chronic hepatitis C. It works for all genotypes and is safe for people with:
- Cirrhosis (even decompensated)
- HIV co-infection
- Chronic kidney disease (including dialysis patients)
- History of liver transplant
- Previous treatment failure with other DAAs
It’s not for people with severe liver failure requiring transplant-those cases need different management. And while it’s safe in pregnancy, data is still limited. Doctors usually wait until after delivery unless the mother’s liver is rapidly failing.
Side Effects and What to Expect
Most people feel fine on velpatasvir. The most common side effects are mild: headache (12%), fatigue (9%), and nausea (6%). Less than 1% stop treatment because of side effects. That’s a huge contrast to older treatments, where up to 40% of patients couldn’t tolerate interferon.
One rare but serious risk: reactivation of hepatitis B. If you’ve ever had hepatitis B, even if you cleared it decades ago, velpatasvir can wake it up. That’s why doctors test for hepatitis B surface antigen before starting treatment. If it’s positive, they add an antiviral like tenofovir to prevent flare-ups.
How It Compares to Other Treatments
| Treatment | Duration | Genotype Coverage | Cure Rate | Key Limitation |
|---|---|---|---|---|
| Velpatasvir + Sofosbuvir (Epclusa) | 8-12 weeks | All 6 | 95-98% | Cost in high-income countries |
| Glecaprevir/Pibrentasvir (Mavyret) | 8 weeks | All 6 | 96-99% | Not for severe kidney disease |
| Elbasvir/Grazoprevir (Zepatier) | 12 weeks | Genotypes 1, 4 | 94-97% | Doesn’t work for genotypes 2, 3, 5, 6 |
| Daclatasvir + Sofosbuvir | 12 weeks | Genotypes 1-6 | 90-95% | Requires multiple pills, drug interactions |
Epclusa isn’t the most expensive option anymore. But Mavyret (glecaprevir/pibrentasvir) is slightly more effective in some groups and has fewer drug interactions. Still, Epclusa remains the go-to for patients with kidney issues or those who’ve failed other treatments.

What’s Next for Velpatasvir?
Researchers are now testing velpatasvir in combination with ultra-long-acting antivirals that could lead to monthly or even quarterly dosing. One 2025 Phase 2 trial used a new injectable formulation of velpatasvir paired with a long-acting sofosbuvir variant. Patients received one shot every 8 weeks-and 92% cleared the virus after two doses.
That could be a game-changer for homeless populations, people with substance use disorders, or those who struggle to take daily pills. Imagine a single clinic visit curing hepatitis C for good.
Another frontier: curing hepatitis C in children under 6. Current guidelines don’t yet approve Epclusa for kids under 6, but new pediatric trials are showing promising results. By 2026, that age limit may drop to 3 years old.
How to Get Started
If you think you might have hepatitis C-especially if you were born between 1945 and 1965, used injection drugs, got a blood transfusion before 1992, or have unexplained liver enzyme spikes-get tested. A simple blood test can detect the virus in minutes.
If you’re diagnosed, ask your doctor about Epclusa. Most insurance plans cover it. If you’re uninsured, patient assistance programs from the manufacturer offer it for free. Generic versions are available through international pharmacies for under $100 if you’re outside the U.S.
There’s no shame in having hepatitis C. It’s not about lifestyle choices-it’s about exposure. And now, it’s curable.
Is velpatasvir the same as sofosbuvir?
No. Velpatasvir and sofosbuvir are two different drugs that work together. Sofosbuvir blocks the virus’s ability to copy its RNA. Velpatasvir blocks the NS5A protein, stopping the virus from assembling new particles. Together, they hit the virus at two critical points, making it nearly impossible to survive treatment.
Can you drink alcohol while taking velpatasvir?
It’s best to avoid alcohol. While velpatasvir doesn’t interact directly with alcohol, drinking can worsen liver damage-especially if you already have cirrhosis. Even after you’re cured, your liver needs time to heal. Stopping alcohol improves recovery and reduces your risk of liver cancer later.
How long does it take for velpatasvir to work?
The virus usually drops below detectable levels within 2-4 weeks. But you must finish the full course-8 or 12 weeks-no matter how you feel. Stopping early increases the chance of the virus coming back. After treatment ends, your doctor will test your blood again 12 weeks later to confirm the cure.
Can hepatitis C come back after velpatasvir treatment?
It’s rare-less than 3% of people relapse. Most of those cases happen in people with advanced cirrhosis or who didn’t finish treatment. If the virus returns, it’s usually not because velpatasvir failed. It’s because the person was reinfected, often through ongoing injection drug use. That’s why harm reduction support is part of successful treatment.
Is velpatasvir safe for older adults?
Yes. In fact, older adults (over 65) often respond better than younger patients. Their immune systems are less likely to fight the treatment, and they’re less likely to have drug interactions if they’re not on multiple medications. The biggest concern is kidney function, which naturally declines with age. Doctors check creatinine levels before prescribing and adjust the dose if needed.
Do I need to get tested again after being cured?
Yes. Even after you’re cured, you should get liver health checks every 1-2 years, especially if you had cirrhosis. The virus is gone, but the damage it caused may still raise your risk of liver cancer. You can also get reinfected if you’re exposed again-so avoid sharing needles, razors, or toothbrushes.
Final Thoughts
Velpatasvir didn’t just make hepatitis C treatable-it made it disappear. What was once a lifelong diagnosis is now a 12-week pill. The science is solid. The access is improving. And the cure rate? Higher than most cancer treatments.
The next challenge isn’t the drug. It’s reaching the people who still don’t know they’re infected. In the U.S., nearly half of those with hepatitis C haven’t been diagnosed. Globally, it’s over 80%. Testing, education, and reducing stigma are the next frontiers.
For anyone reading this-whether you’re a patient, a caregiver, or just curious-know this: hepatitis C is no longer a death sentence. It’s a solvable problem. And velpatasvir is one of the most powerful tools we’ve ever had to solve it.
 Spinal Stenosis and Neurogenic Claudication: How to Recognize Symptoms and Choose the Right Treatment
Spinal Stenosis and Neurogenic Claudication: How to Recognize Symptoms and Choose the Right Treatment
 How to Buy Cheap Generic Metformin Online Safely
How to Buy Cheap Generic Metformin Online Safely
 Epivir (Lamivudine) vs Other HIV Drugs: Detailed Comparison
Epivir (Lamivudine) vs Other HIV Drugs: Detailed Comparison
 9 Alternatives in 2025 to Hydromorphone: Smarter Pain Relief Options
9 Alternatives in 2025 to Hydromorphone: Smarter Pain Relief Options
 Nebulizers vs. Inhalers: Which One Really Works Better for Asthma and COPD?
Nebulizers vs. Inhalers: Which One Really Works Better for Asthma and COPD?
Hope NewYork
November 5, 2025 AT 03:50so yeah i got cured with epclusa last year but now my insurance is trying to deny my follow-up liver scan like it’s some luxury spa treatment. they said ‘you’re cured so why spend money?’ like my liver didn’t just survive a war zone. it needs checkups. dumbasses.
Bonnie Sanders Bartlett
November 5, 2025 AT 10:21I work at a community clinic in rural Ohio and we’ve cured over 200 people with Epclusa since 2022. No specialists needed. No waiting lists. Just a blood test, a prescription, and a little patience. People cry when they hear they’re cured. Not because they’re relieved-they’re mad it took this long. We need more of this everywhere.
Melissa Delong
November 5, 2025 AT 11:58Let me ask you this: if velpatasvir is so miraculous, why did the FDA approve it without long-term cancer risk data? And why are pharmaceutical companies quietly lobbying to keep generics out of the U.S. market? This isn’t medicine-it’s a profit-driven illusion wrapped in a success story. The cure rate looks good… until you dig into the fine print.
Marshall Washick
November 6, 2025 AT 17:26I’ve been a nurse for 22 years. I watched people die from interferon. I held hands while they vomited for weeks. Then came Epclusa. One patient, 71, diabetic, cirrhosis, never left his house for 10 years. Took the pills. Got cured. Walked into the pharmacy three months later to buy his first pack of cigarettes since 1998. Smiled. Said, ‘I didn’t think I’d ever see the day.’ That’s what this is. Not science. Humanity.
Rohan Puri
November 8, 2025 AT 15:53Mandeep Singh
November 8, 2025 AT 22:30India giving away cures? Don’t be fooled. They’re exporting cheap generics because they don’t care about their own people’s health. The real story is how the U.S. is still leading medical innovation while others just copy and sell. We built this. They just stole it.
Abha Nakra
November 10, 2025 AT 20:54Just wanted to add something important-many people in rural India and Kenya can’t even get the generic pills because of poor logistics. The drug works, but the system doesn’t. We need mobile clinics, not just cheap drugs. I’ve helped distribute Epclusa generics in Bihar. People walk 20km to get it. We need to meet them where they are.
Neal Burton
November 12, 2025 AT 19:27How quaint. A pill that cures a disease? How… pedestrian. The real breakthrough would be gene-editing the virus out of the human genome entirely. Velpatasvir is a Band-Aid on a bullet wound. The pharmaceutical-industrial complex celebrates mediocrity because it’s profitable. True progress is measured in decades, not weeks.
Tamara Kayali Browne
November 13, 2025 AT 13:5797% cure rate? That’s statistically impressive, but it ignores the confounding variables: selection bias in clinical trials, attrition in real-world settings, and the fact that many patients with advanced cirrhosis were excluded from early data. The headline is misleading. We’re not eradicating hepatitis C-we’re just reclassifying it as a managed condition with a high compliance burden.
John Rendek
November 13, 2025 AT 19:07If you’re reading this and you’re scared to get tested-don’t be. This isn’t a moral failure. It’s a medical one. And it’s fixable. One pill. One month. One chance to live like you never had it. Get tested. Then get cured. You’ve got nothing to lose but the ghost in your liver.