When you hear about a new cancer drug in the news, it’s easy to think it’s just another pill that might help. But behind every successful treatment is a carefully designed clinical trial-and the key to who gets in isn’t just about age or stage of cancer anymore. It’s about biomarkers. These are biological signals in your body-like specific genes, proteins, or cell markers-that tell doctors whether a drug will likely work for you. If you’re considering a clinical trial for cancer, understanding how biomarkers and inclusion criteria work isn’t just helpful-it could change your treatment path.
What Are Biomarkers, Really?
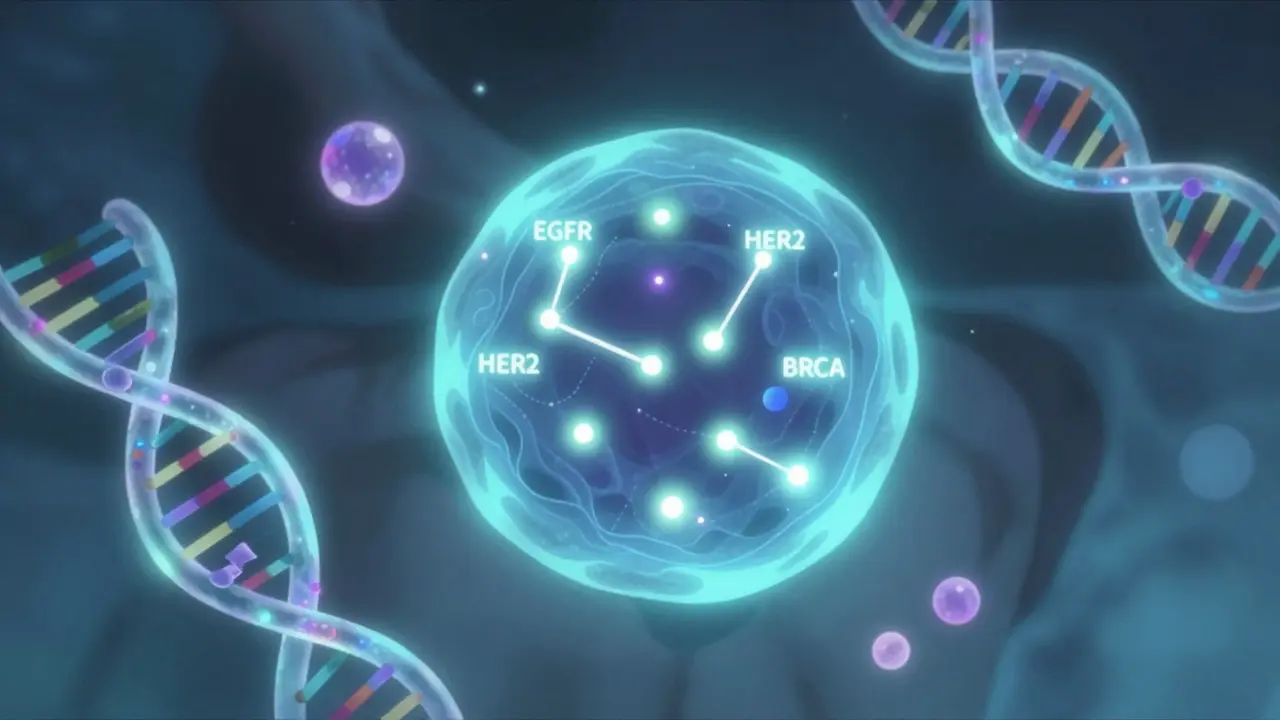
Biomarkers aren’t magic. They’re measurable things in your body that give clues about what’s happening inside. Think of them like a car’s dashboard. The check engine light? That’s a biomarker. It doesn’t tell you exactly what’s broken, but it tells you something’s off. In cancer, biomarkers might be a mutation in the BRCA gene, high levels of PD-L1 protein on tumor cells, or the presence of a specific protein called HER2. These aren’t just labels-they’re signals that help match patients to drugs that target those exact changes. The FDA recognizes seven types of biomarkers, but for clinical trials, three matter most: predictive, prognostic, and pharmacodynamic. Predictive biomarkers tell you if a drug will work-like testing for EGFR mutations before giving osimertinib to lung cancer patients. Prognostic biomarkers tell you how aggressive the cancer is, regardless of treatment. And pharmacodynamic biomarkers show if the drug is doing what it’s supposed to-like lowering a certain protein level after treatment. In cancer trials, predictive biomarkers are the stars. They’re the reason some patients respond dramatically while others don’t.Why Inclusion Criteria Are Now More Precise Than Ever
In the past, clinical trials often included patients based on broad categories: “stage IV lung cancer,” “breast cancer after chemo,” “over 18.” That led to trials where only 10-15% of participants actually responded to the drug. It was like giving everyone in a city a key to one lock-most keys won’t fit. Today, inclusion criteria are laser-focused. A trial might now say: “Adults with metastatic non-small cell lung cancer, confirmed EGFR exon 19 deletion, no prior targeted therapy, ECOG performance status 0-1.” That’s not just a mouthful-it’s precision. This kind of criteria cuts down trial failures. Between 2011 and 2020, trials using biomarkers had nearly a 50% success rate in Phase 2, compared to just 27% for those that didn’t. That’s an 84% improvement. Why does this matter to you? Because if you don’t have the right biomarker, you won’t be eligible-even if you have the same cancer type as someone else. That’s frustrating, but it’s also better for everyone. You’re not wasting months on a drug that won’t work. And the drug developers aren’t wasting money testing on people who won’t benefit.How Biomarker Testing Works-and Why It’s Not Always Simple
Getting tested isn’t as simple as walking into a lab and asking for a test. First, your tumor tissue must be collected-usually during a biopsy or surgery. That tissue is sent to a specialized lab, often CLIA-certified, where they run tests like next-generation sequencing or immunohistochemistry. These tests can take 1-3 weeks. Some centers now use liquid biopsies-blood tests that look for tumor DNA floating in your bloodstream. That’s faster and less invasive, and it’s growing fast: 31% of Phase 2+ cancer trials in 2023 used them, up from just 9% in 2020. But here’s the catch: not all labs are equal. One lab might report a mutation as “positive,” while another says “uncertain.” That’s why standardization is critical. In 82% of multi-center trials, sponsors report inconsistent testing protocols across sites. That’s why many trials now use central labs to handle all biomarker testing. If you’re enrolling in a trial, ask: “Who runs the test? Is it the same lab for everyone?” Also, timing matters. Biomarker status can change over time. A tumor that was HER2-negative at diagnosis might become HER2-positive after treatment. That’s why some newer trials allow retesting during the study. If your cancer progresses, ask if you’re eligible for retesting-even if you were turned down before.
Real-World Impact: When Biomarkers Make a Difference
Take HER2-positive breast cancer. Before biomarkers, treatments were blunt. Now, drugs like trastuzumab and neratinib are only given to patients with confirmed HER2 overexpression. In one trial, response rates jumped from 12% in unselected patients to 32% in those with the biomarker. That’s not just a number-it’s more time, more hope, fewer side effects from drugs that wouldn’t have worked. In lung cancer, patients with ALK or ROS1 mutations now live years longer than they did a decade ago-not because the drugs are stronger, but because they’re targeted. A 2022 study showed that biomarker-driven lung cancer trials recruited patients 28 days faster than traditional ones, because the right people were found sooner. Even in rare cancers, biomarkers are opening doors. A child with a specific NTRK gene fusion can now get a drug approved for any cancer type that has that mutation-not just the one it started in. That’s the power of biomarkers: they shift the focus from where the cancer is to what’s driving it.The Hidden Challenges: Why Not Everyone Gets In
It’s not all smooth sailing. Biomarker testing requires infrastructure. Many community hospitals don’t have the equipment, staff, or partnerships to run advanced tests. That means patients in rural areas or smaller clinics often get left out. A 2023 survey found that 68% of trial sites reported delays of 7-14 days for biomarker results-long enough to lose patients who can’t wait. There’s also a geographic gap. Some biomarkers are rare in certain populations. For example, the HLA-A*02:01 marker, used in some cell therapies, appears in 40-50% of Europeans but only 17-48% of North Americans. That means a trial that works well in Germany might struggle to find enough patients in Boston. And then there’s cost. Biomarker tests can run from $500 to $5,000. Insurance doesn’t always cover them for research purposes. Some trials pay, but not all. If you’re considering a trial, ask: “Who pays for the biomarker test? What if it’s not covered?”
What You Can Do: Navigating the System
If you’re exploring clinical trials, here’s what to do:- Ask your oncologist for a biomarker test if you haven’t had one. Even if you’re not ready for a trial now, knowing your biomarker profile helps for the future.
- Don’t assume you’re ineligible. Some trials use multiple biomarkers or allow retesting. Ask if there’s a trial that fits your profile-even if it’s not the one you first heard about.
- Request a copy of your biomarker report. Keep it. It’s your medical record. You might need it for future trials or second opinions.
- Use trusted resources like ClinicalTrials.gov. Filter by “biomarker” and your cancer type. Look for trials that mention “molecular profiling” or “genomic testing.”
- Ask about central testing. If the trial uses a central lab, ask how long results take. If it’s local, ask if they’ve done the test before and how reliable it is.
The Future: Where This Is All Headed
The field is moving fast. By 2025, 65% of new cancer trials are expected to use multi-omic biomarker panels-combining DNA, RNA, protein, and even immune system data into one profile. AI is already being used to find new biomarkers from old data. And in 2023, the FDA streamlined its biomarker qualification process, cutting review time from 24 to 18 months and raising approval rates to 73%. The goal isn’t just to find drugs that work-it’s to find the right drug for the right person, every time. That’s precision medicine. And it’s no longer the future. It’s the standard.By 2030, experts predict 80% of clinical trials will rely on biomarkers. That means more targeted treatments, fewer side effects, and better outcomes. But it also means you need to be proactive. Know your biomarkers. Ask questions. Advocate for yourself. Your cancer is unique-and so should be your treatment.
What are the most common biomarkers used in cancer clinical trials?
The most common biomarkers in cancer trials include EGFR, ALK, ROS1, and KRAS mutations in lung cancer; HER2 overexpression in breast and gastric cancers; BRCA1/2 mutations in ovarian and breast cancers; PD-L1 expression for immunotherapy eligibility; and MSI-H or dMMR status for colorectal and other solid tumors. These are well-established, FDA-recognized markers that directly guide treatment decisions in targeted therapy and immunotherapy trials.
Can I be denied a clinical trial even if I have the right type of cancer?
Yes. Having the right cancer type isn’t enough. You must also meet the biomarker criteria. For example, a trial for an EGFR inhibitor will only accept patients with confirmed EGFR mutations-even if they have stage IV lung cancer. Other factors like lab values, prior treatments, organ function, and overall health also play a role. Biomarker status is often the gatekeeper.
How long does biomarker testing usually take?
Standard tissue-based testing typically takes 10 to 14 days. Liquid biopsies can be faster-sometimes as quick as 5-7 days. But delays happen due to sample shipping, lab backlogs, or the need for repeat testing. Some trials use central labs to speed this up, while others rely on local hospitals, which can add weeks. Always ask the trial coordinator for their estimated timeline.
Are biomarker tests covered by insurance?
Coverage varies. If the test is part of standard clinical care (like testing for HER2 in breast cancer), insurance usually covers it. But if it’s for research purposes-like identifying eligibility for a trial-it often isn’t covered. Many trials cover the cost of the test as part of enrollment. Always ask the trial team: “Who pays for this test?” and get it in writing.
What if my biomarker test comes back negative? Does that mean no options?
Not at all. A negative result for one biomarker doesn’t mean you’re out of options. Many trials now test for multiple biomarkers at once. You might be eligible for a trial targeting a different mutation, or one that combines therapies. Some trials are designed for patients with no known biomarkers-these are called “basket trials” or “umbrella trials.” Ask your oncologist to re-evaluate your case with newer testing methods, especially if your cancer has progressed.
 Coronary Artery Disease and Mental Health: The Impact on Emotional Well-being
Coronary Artery Disease and Mental Health: The Impact on Emotional Well-being
 Pharmacy Counseling: What to Learn When Picking Up Generics
Pharmacy Counseling: What to Learn When Picking Up Generics
 The Link Between Bimatoprost and Dry Eye Syndrome
The Link Between Bimatoprost and Dry Eye Syndrome
 How to Choose OTC Eye Drops for Allergies, Dryness, and Redness
How to Choose OTC Eye Drops for Allergies, Dryness, and Redness
 Coronary Calcium Score: What CT Scans Reveal About Plaque Buildup in Your Arteries
Coronary Calcium Score: What CT Scans Reveal About Plaque Buildup in Your Arteries
bob bob
January 4, 2026 AT 00:17Man, I never realized how much goes into this stuff. My aunt just got into a trial last year and they spent weeks just testing her tumor. She was so frustrated, but now she’s on a drug that’s actually working. I wish more people knew this was even a thing.
Vicki Yuan
January 5, 2026 AT 04:19One thing that’s often overlooked: biomarker testing isn’t just about eligibility-it’s about dignity. Being told ‘this drug won’t work for you’ is painful, but being told ‘here’s exactly what will’? That’s empowerment. Precision medicine isn’t just science; it’s respect.
Uzoamaka Nwankpa
January 6, 2026 AT 07:21They keep saying ‘it’s better for everyone’ but what about the people who get left behind? Rural hospitals can’t afford these tests. People die waiting. This isn’t progress-it’s a luxury for the lucky.
Chris Cantey
January 6, 2026 AT 18:45Biological signals, really? Sounds like modern alchemy. They take a few proteins, slap a label on them, and call it science. But cancer’s a system-wide collapse-not a broken lightbulb. You can’t reduce a living organism to a dashboard warning. We’re just rearranging deck chairs on the Titanic.
Abhishek Mondal
January 7, 2026 AT 01:01Let’s be real: the entire ‘biomarker-driven’ paradigm is a corporate R&D strategy disguised as medical progress. The FDA ‘streamlined’ approval? That’s just regulatory capture. And don’t get me started on ‘central labs’-they’re profit centers. The real innovation? Monetizing genetic data under the guise of ‘precision.’
Oluwapelumi Yakubu
January 8, 2026 AT 09:39Yo, this whole biomarker thing? It’s like finding the right key to unlock your body’s secret vault. I’ve seen people go from ‘terminal’ to ‘living with cancer’ because someone finally looked at their DNA and said, ‘Ah! This is your thing.’ It’s not magic-it’s just the universe whispering, ‘Here’s your blueprint.’ And yeah, the wait sucks, the cost sucks-but when it works? Man, it’s like the universe high-fives you.
Terri Gladden
January 8, 2026 AT 18:39OMG I just found out my mom’s biomarker test got lost in the mail!! Like, what?? They sent it to the wrong lab and now they want to re-do the biopsy?? I’m crying. This system is broken. I hate this. Someone please fix this.
Jennifer Glass
January 9, 2026 AT 09:47I’ve been following this since my brother’s diagnosis. What struck me most isn’t the science-it’s the silence. Most patients don’t know to ask for biomarker testing until it’s too late. Oncologists are overwhelmed. We need better patient education, not just better tech. A simple pamphlet in waiting rooms could save lives. Why isn’t that standard?
Joseph Snow
January 10, 2026 AT 01:46Let’s not pretend this is about patient care. The pharmaceutical industry is using biomarkers to cherry-pick the most responsive patients, then charging $500,000 per year for drugs that only work on 12% of the population. The rest? They’re collateral. This isn’t medicine-it’s a financial algorithm with a stethoscope.