Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
- by Lysander Beaumont
- Jan, 16 2026
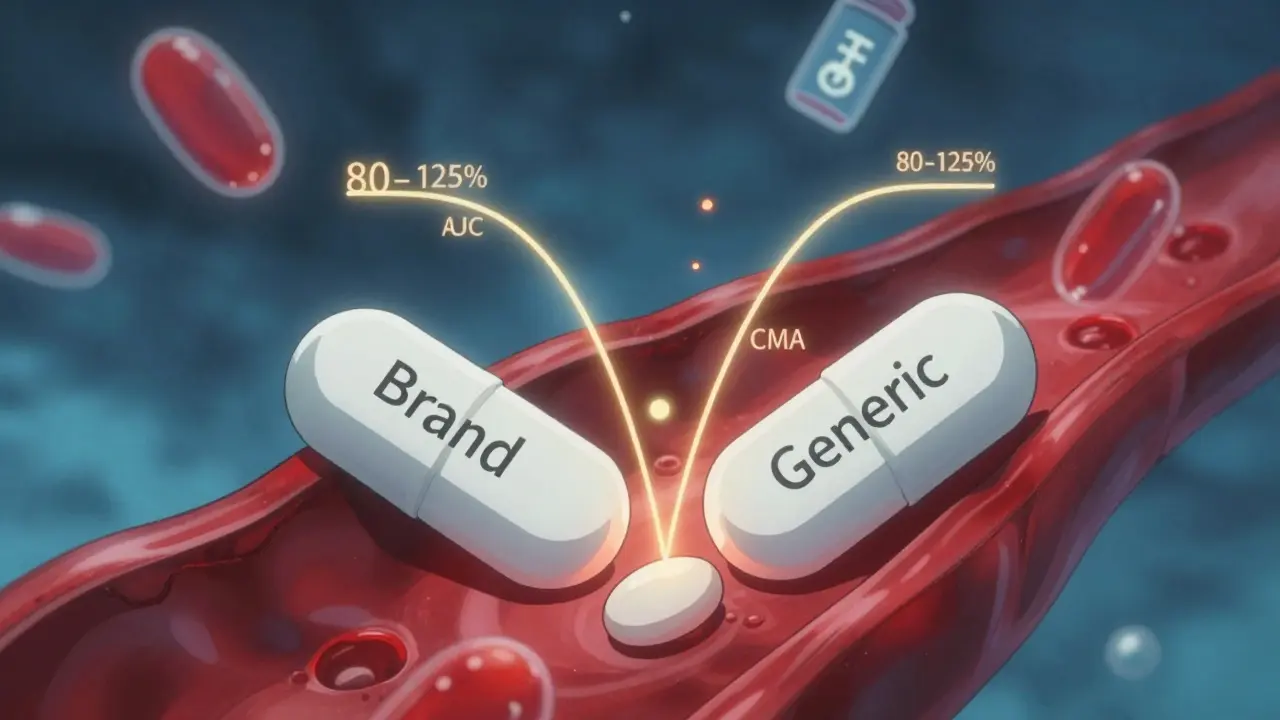
The FDA requires generic drug manufacturers to prove bioequivalence through strict pharmacokinetic studies, ensuring their products match the brand-name drug in absorption and effectiveness. Learn how the 80-125% rule, biowaivers, and product-specific guidelines ensure safety.
Read More